14/03/2022 - 13:00 - 12:00
Add To Calendar
2022-03-14 12:00:00
2022-03-14 13:00:00
קולוקוויום מחלקתי 14.03.2022
הקולוקויום יתקיים ביום שני 14/03/2022 בשעה 12:00 בחדר הסמינרים המחלקתי (211/112)
The Department of Chemistry Weekly Seminar will take place on Monday 14/03/22, 12:00pm in the Department seminar room 211/112.
Joshua Baraban,
Dept. of Chemistry, BGU
Ring opening and tunneling inversion in the cyclopropyl radical and cation
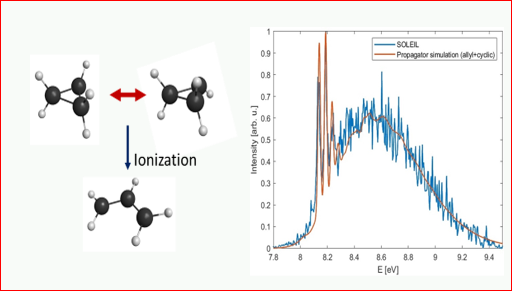
We report spectroscopic and theoretical studies of the cyclopropyl radical and cation (c-C3H5). The cation especially is unstable towards ring-opening to allylic geometries, and the radical exhibits inversion tunneling by the α-H atom through the C-C-C ring plane. These large amplitude motion phenomena complicate the photoionization (and other) spectra and the determination of properties that are of interest due to ring strain in this fundamental cyclic radical. Through a multiscale reduced-dimension ab initio description of the potential energy surfaces (PES) of both the radical and the cation, the rotational and ionization spectra of the radical are simulated using advanced perturbative and variational rovibrational treatments.
Due to the large energy difference between the allylic equilibrium geometry and the unstable cyclic configuration on the cation PES, propagator-based methods that avoid the construction of cationic vibronic eigenstates were used to simulate the ionization spectrum. The results of our simulations compare well with both experimental photoionization data from the literature and new mass-selected threshold photoelectron measurements performed at the SOLEIL synchrotron. Further computational efforts are underway to improve the simulations.
These results will allow the determination of important thermochemical quantities, such as the C-H bond dissociation energy of cyclopropane. This work illustrates how advanced computational methods and experimental techniques can be utilized together to explore fundamental yet challenging chemical problems.
חדר הסמינרים 211/112
Department of Chemistry
chemistry.office@biu.ac.il
Asia/Jerusalem
public
מיקום
חדר הסמינרים 211/112
הקולוקויום יתקיים ביום שני 14/03/2022 בשעה 12:00 בחדר הסמינרים המחלקתי (211/112)
The Department of Chemistry Weekly Seminar will take place on Monday 14/03/22, 12:00pm in the Department seminar room 211/112.
Joshua Baraban,
Dept. of Chemistry, BGU
Ring opening and tunneling inversion in the cyclopropyl radical and cation
We report spectroscopic and theoretical studies of the cyclopropyl radical and cation (c-C3H5). The cation especially is unstable towards ring-opening to allylic geometries, and the radical exhibits inversion tunneling by the α-H atom through the C-C-C ring plane. These large amplitude motion phenomena complicate the photoionization (and other) spectra and the determination of properties that are of interest due to ring strain in this fundamental cyclic radical. Through a multiscale reduced-dimension ab initio description of the potential energy surfaces (PES) of both the radical and the cation, the rotational and ionization spectra of the radical are simulated using advanced perturbative and variational rovibrational treatments.
Due to the large energy difference between the allylic equilibrium geometry and the unstable cyclic configuration on the cation PES, propagator-based methods that avoid the construction of cationic vibronic eigenstates were used to simulate the ionization spectrum. The results of our simulations compare well with both experimental photoionization data from the literature and new mass-selected threshold photoelectron measurements performed at the SOLEIL synchrotron. Further computational efforts are underway to improve the simulations.
These results will allow the determination of important thermochemical quantities, such as the C-H bond dissociation energy of cyclopropane. This work illustrates how advanced computational methods and experimental techniques can be utilized together to explore fundamental yet challenging chemical problems.