Seminar: Rapid, Modular and Stereodivergent Synthesis of Acyclic 1,4-Stereocenters: Experiment Design, Scope and Mechanistic Insight
S E M I N A R
Monday 18/11/19, 12:00 pm
Building 211, seminar room
SPEAKER:
Dr. David Pierrot
Technion – Israel Institute of Technology
TOPIC:
Rapid, Modular and Stereodivergent Synthesis of Acyclic 1,4-Stereocenters: Experiment Design, Scope and Mechanistic Insight
The preparation of acyclic molecules bearing multiple elements of complexity (unsaturations, centers of chirality) has been a great challenge for synthetic chemists when these elements are located in a close vicinity.[1] While many methods enable the enantioselective preparation of specific templates, stereodiversity can mostly be accessed through long linear sequences. Recent stereodivergent preparations of 1,2-contiguous stereocenters received great interest from the community.[2],[3] In order to pursue the chemical space’s exploration,[4] more rapid and efficient preparations of any stereoisomer of a given stereofamily are required.
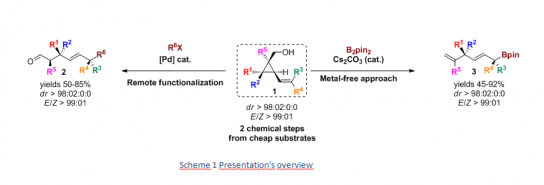
Owing to a specific substrate design (Scheme 1), we could access acyclic 1,4-stereocenters in a stereodivergent manner through two different approaches. A palladium-catalyzed remote functionalization approach leads to the 1,2,5-stereocenter family 2.[5] A metal-free catalytic diboration strategy afforded the preparation of 1,4-stereocenter family 3 containing an allylic boronic ester functional group.[6] Both methods are versatile and enable the preparation of both tertiary or quaternary stereocenters in a stereodivergent approach.
[1] D. Pierrot, I. Marek, Angew. Chem. Int. Ed. 2019, 58, 2-16.
[2] S. Krautwald, D. Sarlah, M. A. Schafroth, E. M. Carreira, Science 2013, 340, 1065.
[3] D. Kaldre, I. Klose, N. Maulide, Science 2018, 361, 664.
[4] J.-L. Reymond, Acc. Chem. Res. 2015, 48, 722.
[5] J. Bruffaerts, D. Pierrot, I. Marek, Nature Chem. 2018, 10, 1164.
[6] Unpublished results
Last Updated Date : 13/11/2019



