Special Seminar: From Simplicity to Complexity: Strategic Design & Applications
S P E C I A L S E M I N A R
Monday 3/06/19, 12:00 am
Building 211, seminar room 112
SPEAKER:
Dr. Zackaria (Zack) Nairoukh
TOPIC:
From Simplicity to Complexity: Strategic Design & Applications
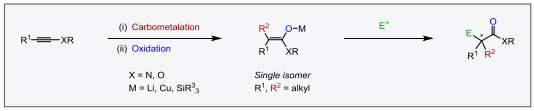
The modular assembly of complex organic molecules is one of the most difficult challenges in organic chemistry. New approaches are constantly needed for the development of selective and efficient strategies that can facilitate the discovery and development of life-changing medicines, agrochemicals and materials. For instance, the formation of enantiomerically pure quaternary carbon centers α to a carbonyl group in acyclic systems remains elusive and has been the focus of only a few studies. The main problem that limits the formation of this stereocenter is the difficult preparation of stereodefined trisubstituted enolates with different alkyl groups. To circumvent this issue, we developed an efficient strategy for the formation of stereodefined trisubstituted copper enolates from simple alkynes by a carbometalation/oxidation sequence and their application in several transformations.1-2 Additionally, the reactivity of the crossponding trisubstituted silyl enol ethers in Mukaiyama aldol,3-4 electrophilic fluorination5 and oxidation reactions6 was investigated.
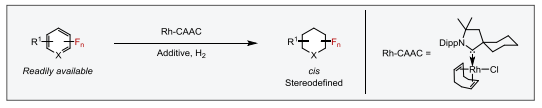
In a similar manner, we addressed other challenging problems in organic chemistry, such as those associated with chemoselectivity in arene hydrogenation, e.g. reduction of the aromatic ring without losing functional groups through further hydrogenation or hydrodefunctionalization.7 In particular, we approached the hydrogenation of fluoroarenes8 and fluoropyridines9 towards the generation of all-cis-(multi)fluorinated carbo- and heterocycles. The preparation of the latter species usually requires tedious multistep syntheses with low yields and diastereoselectivities. Accordingly, we were able to reduce the aromatic rings with almost no hydrodefluorination side products. This has enabled the preparation of a library of fluorinated aliphatic motifs in high yields and with excellent diastereoselectivities.
References:
[1] Minko, Y., Pasco, M., Lercher, L., Botoshansky, M., Marek, I. Nature 2012, 490, 522.
[2] Nairoukh, Z., Kumar, G. N., Minko, Y., Marek, I. Chem. Sci. 2017, 8, 627.
[3] Nairoukh, Z., Marek, I. Angew. Chem. Int. Ed. 2015, 54, 14393.
[4] Haimov, E., Nairoukh, Z., Shterenberg, A., Berkovitz, T., Jamison, T. F., Marek, I. Angew. Chem. Int. Ed. 2016, 55, 5517.
[5] Huang, J. Q., Nairoukh, Z., Marek, I. Org. Biomol. Chem. 2018, 16, 1079.
[6] Huang, J. Q., Nairoukh, Z., Marek, I. Eur. J. Org. Chem. 2018, 614.
[7] Wiesenfeldt, M., Nairoukh, Z., Dalton, T., Glorius, F. Angew. Chem. Int. Ed. 2019, accepted.
[8] Wiesenfeldt, M., Nairoukh, Z., Li, W., Glorius, F. Science 2017, 357, 908.
[9] Nairoukh, Z., Wollenburg, M., Schlepphorst, C., Bergander, K., Glorius, F. Nat. Chem. 2019, 11, 264.
Last Updated Date : 23/05/2019



