26/11/2018 - 13:00 - 12:00
Add To Calendar
2018-11-26 12:00:00
2018-11-26 13:00:00
Seminar: Novel π-Conjugated Materials – Planar and Twisted
S E M I N A R
Monday 26/11/18, 12:00 pm
Building 211, seminar room
SPEAKER:
Dr. Ori Gidron,
Institute of Chemistry, The Hebrew University of Jerusalem, Israel.
TOPIC:
Novel π-Conjugated Materials – Planar and Twisted
For organic electronic materials which composed of π-conjugated backbones, planarity is a crucial factor determining their electronic and optical properties. In this talk, I will discuss two examples of planar and non-planar systems: planar oligofurans and twisted acenes.
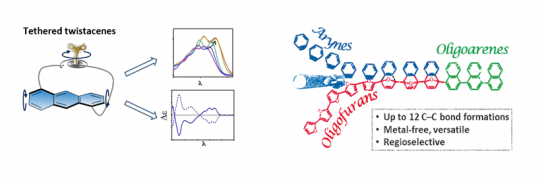
In the first part, we demonstrate the transformation of planar oligofurans through sequential Diels–Alder cycloaddition reactions to provide oligoarenes in two chemical steps, regardless of the oligomer length. By this method, oligonaphthalenes containing up to six units were obtained in high yield through the formation of up to 12 new C−C bonds. The versatility of this method was demonstrated for various polyaromatic hydrocarbons.[1]
In the second part, I will introduce a series of twisted acenes having an anthracene backbone diagonally tethered by an n-alkyl bridge, which induces a twist of various angles. This allows us to systematically monitor the effect of twisting on electronic and optical properties. We find that absorption is bathochromically shifted with increasing twist, while fluorescence quantum efficiency drops dramatically. The tethered twistacenes were isolated to their enantiomerically pure form, displaying strong chiroptical properties and anisotropy factor (g- value). No racemization was observed even upon prolonged heating, rendering these tethered twistacenes suitable as enantiopure helical building units for π-conjugated backbones. [2]
Reference
S. Phatangare, L. J. W. Shimon and O. Gidron Angew. Chem. Int. Ed. 2017, 56, 13601–13605.
A. Bedi, L. J. W. Shimon, and O. Gidron J. Am. Chem. Soc. 2018, 140, 8086.
Abstract
Department of Chemistry
chemistry.office@biu.ac.il
Asia/Jerusalem
public
S E M I N A R
Monday 26/11/18, 12:00 pm
Building 211, seminar room
SPEAKER:
Dr. Ori Gidron,
Institute of Chemistry, The Hebrew University of Jerusalem, Israel.
TOPIC:
Novel π-Conjugated Materials – Planar and Twisted
For organic electronic materials which composed of π-conjugated backbones, planarity is a crucial factor determining their electronic and optical properties. In this talk, I will discuss two examples of planar and non-planar systems: planar oligofurans and twisted acenes.
In the first part, we demonstrate the transformation of planar oligofurans through sequential Diels–Alder cycloaddition reactions to provide oligoarenes in two chemical steps, regardless of the oligomer length. By this method, oligonaphthalenes containing up to six units were obtained in high yield through the formation of up to 12 new C−C bonds. The versatility of this method was demonstrated for various polyaromatic hydrocarbons.[1]
In the second part, I will introduce a series of twisted acenes having an anthracene backbone diagonally tethered by an n-alkyl bridge, which induces a twist of various angles. This allows us to systematically monitor the effect of twisting on electronic and optical properties. We find that absorption is bathochromically shifted with increasing twist, while fluorescence quantum efficiency drops dramatically. The tethered twistacenes were isolated to their enantiomerically pure form, displaying strong chiroptical properties and anisotropy factor (g- value). No racemization was observed even upon prolonged heating, rendering these tethered twistacenes suitable as enantiopure helical building units for π-conjugated backbones. [2]
Reference
- S. Phatangare, L. J. W. Shimon and O. Gidron Angew. Chem. Int. Ed. 2017, 56, 13601–13605.
- A. Bedi, L. J. W. Shimon, and O. Gidron J. Am. Chem. Soc. 2018, 140, 8086.
Abstract