
Prof. Gerardo Byk
Design of mono dispersed nanoparticles for sensing, multiplexing, drug delivery, gene delivery.
Discovery of targeting peptides
CV
Curriculum vitae
Academic Background
|
From-To |
Institution |
Area of specialization |
Degree |
|
1986-1990 |
The Hebrew University of Jerusalem |
Medicinal Chemistry |
Ph. D. |
|
1984-1986 |
The Hebrew University of Jerusalem |
Organic Chemistry |
M. Sc. |
|
1980-1983 |
The Hebrew University of Jerusalem |
Chemistry |
B. Sc. |
Previous Employment
|
From-To |
Institution |
Research area |
Title |
|
October 2002-present |
Bar Ilan University |
Nanobiotechnology |
Associate Professor |
|
September 1998-2002 |
Bar Ilan University |
Peptidomimetics and Genetic Chemistry |
Senior Lecturer |
|
1996-1998 |
Rhone Poulenc Rorer (France-Paris) |
Genetic Chemistry |
Associate Research Fellow |
|
1994-1996 |
Rhone Poulenc Rorer (France-Paris) |
Genetic Chemistry |
Senior Research Scientist |
|
1992-1994 |
Rhone Poulenc Rorer |
Peptidomimetics |
Research Scientist |
|
1991-1992 |
InstitutPasteur Lyon France |
Peptidomimetics |
Post –Doc. |
Research
Nanobiotechnology, high-throughput technologies for life cell and in vivo screening of small molecules for tissue targeting, tumor targeting.
Non viral vectors for gene delivery and expression of foreign gens for gene therapy.
Organic synthesis of natural products. Development of organic methods.
1.Nanobiotechnology for drug discovery and diagnostics
2.Total synthesis of natural products
3. combinatorial chemistry and combinatorial screening technologies
4. Non viral gene delivery for gene therapy
Combinatorial Chemistry: Towards the chemistry of the 21st century
In the last twenty years, pharmaceutical companies have invested significant efforts in developing robotics and miniaturization for biological screening purposes. As a result of these efforts, the capacity of biologists to perform in vitro high-throughput screening of chemicals for drug discovery was dramatically improved. The main limitation of this new screening technology lies in the capacity of the chemists to furnish biologists with great diversity and number of products.
Traditionally, drug discovery involved the optimization of lead structures, most likely derived from biological sources, through a multi-step process of serial synthesis and screening. This approach is extremely costly, as each compound will have been individually handcrafted in solution by a synthetic chemist. The need to find more cost-effective methods of drug development, combined with the recent advances in robotic screening which enable the testing of hundreds of thousands of products per year, has led pharmaceutical companies to examine combinatorial synthetic strategies as a means of accelerating drug discovery programs, and increasing the chemical diversity of their compound libraries.
Chemists in academia are aware that novel synthetic tools must be developed in order to meet the increasing requirements of the high-throughput biological screening technology. For this purpose, the use of robotics in organic synthesis may significantly improve the diversity of synthetic libraries, and may allow the finding of new organic reactions. The emerging technology, the so-called ‘Combinatorial Chemistry’, is based on the simple premise that the greater the diversity of compounds tested, the better the chance of finding one that can be developed into a drug or other industrial lead. Although it is theoretically not limited to any specific family of chemical species, the scope of this approach is actually limited as a result of the restrained organic reactions available for synthesis on solid supports, and by the limited quantity of available multi-reagent organic reactions. A main goal of the laboratory of peptidomimetics and genetic chemistry will be to discover and develop new tools for extending the scope of combinatorial chemistry, and the application of these new tools to drug discovery.
http://pubs.acs.org/doi/abs/10.1021/cc900156z
The laboratory will focus on the design and delivery of biologically active molecules and complexes for drug research and clinical applications.
Some tasks of the laboratory:
Development of new technologies for automation and miniaturization of organic synthesis: Multicomponent and multistep organic synthesis on solid supports for high throughput synthesis.
http://pubs.acs.org/doi/abs/10.1021/cc900156z
http://pubs.acs.org/doi/abs/10.1021/cc000056p?source=chemport
https://www.thieme-connect.de/DOI/DOI?10.1055/s-2006-932498
http://pubs.acs.org/doi/abs/10.1021/cc049962i?source=chemport
Discovery of molecules with interesting biological activity using screening approaches such as combinatorial chemistry and multiple organic synthesis, and rational approaches starting from the natural substrates, especially from peptide sequences.
http://springer.metapress.com/content/x24u058q453q1034/
Definition of the bioactive conformation of biologically active analogs derived from synthetic molecules, or from natural compounds whose pharmacological characteristics are well known.
Development of integrated nanotechnology both for high-throughput organic synthesis and in vivo biological screening.
A new "phage display-like" synthetic system is generated using 2 mm cross-linked mono-dispersed microspheres bearing a panel of fluorescence tags and peptides that are directly synthesized on the microspheres or small molecules covalently conjugated to them. The new system allows screening mixtures of ligands bound to the microspheres using flow-cytometry analysis of the microspheres after incubation with a live cells model PC-3 cell-line in analogy to phage-display peptide method. The advantage of the proposed system is the possibility of screening non natural peptides, combinatorial libraries and small molecules that cannot be expressed and screened using phage display libraries. Two libraries are screened resulting in peptide DUP-1(1-12)2Ala and small molecule Rak-2 both individually synthesized and validated in an independent binding assay. Finally, the cellular fate of DUP-1(1-12)2Ala in PC-3 cell line is demonstrated using confocal laser scanning microscopy.
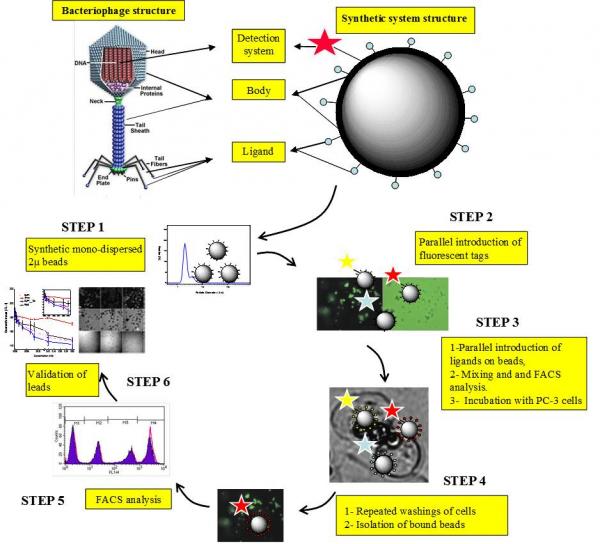
Figure 1. Design of a phage-like synthetic system and steps of the analysis technique. Background microscopy pictures in steps 2, 3 and 4 are real representations of the steps as seen by inverted microscopy.
http://pubs.acs.org/doi/abs/10.1021/cc900156z
http://www.benthamscience.com/open/tooptsj/articles/V005/SI0011TOOPTSJ/17TOOPTSJ.pdf
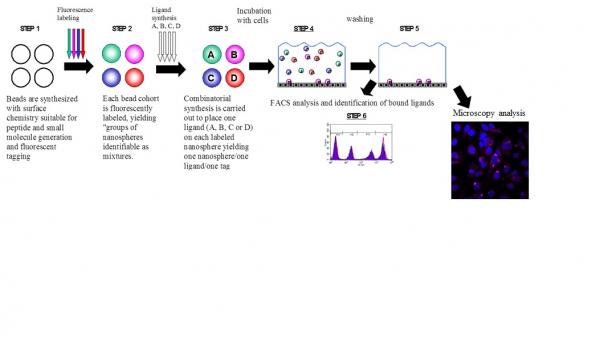
Fig. (1). Steps of live cell screening using a phage-like synthetic system.
Development of novel targeted imaging systems for diagnostics in collaboration with French scientists: https://www.sciencedirect.com/science/article/pii/S0378517311006260
2. Gene therapy
Synthetic DNA delivery agents are of crucial importance for gene therapy as an alternative to viral vectors, since they display potentially less risks in terms of immunogenicity and propagation.
A great deal of work is still needed for obtaining improved gene expression after non-viral gene delivery, especially regarding the in vivo final localization of the DNA complexes. Based on recently published results, the introduction of a targeting moiety into non-viral gene delivery systems promises improved gene expression in the targeted area as result of the accumulation of the complexes.
http://pubs.acs.org/doi/abs/10.1021/bc025567e?source=chemport
The laboratory aims to develop non-viral gene delivery systems specifically targeted to tumors. Specific tumor receptors are of particular interest. Thus, targeting ligands addressed to these receptors will allow a selective localization of the therapeutic gene or drug in the tumor area. Coupling of these ligands to complexes carrying suicide genes will promote the specific elimination of tumor cells, resulting in tumor reduction or elimination.
3- Total synthesis of natural compounds with biological activities
Novel methodologies especially employing microwave energy are being developed in the laboratory for the synthesis of biological relevant natural compounds. The lab designs and develop synthetic methodologies for this aim and characterise the obtained molecules for final validation of the natural compounds structure.
http://www.arkat-usa.org/get-file/39666/
Particular research areas (projects):
1. Nano-biotechnology for drug discovery and diagnostics
2. Total synthesis of natural products
3. Combinatorial chemistry and combinatorial screening technologies
4. Non-viral gene delivery for gene therapy
OPEN POSITION
Bachelor or MSc students in chemistry, biophysics, biology, physics, biotechnology, biomedical engineering from any university or recognized college are asked to apply to Prof. Byk for MSc or PhD positions currently open.
Publications
http://scholar.google.co.il/citations?hl=en&user=IhPZGIAAAAAJ&view_op=list_works&sortby=pubdate
- Eshwaran, L., Kazimirsky, G., Yehuda, R. and Byk, G. A new strategy for nucleic acid delivery and protein expression using biocompatible Nanohydrogels of predefined sizes. Pharmaceutics (IF:5.4, JCR, Q1) 2023, 15(3), 961. https://doi.org/10.3390/pharmaceutics15030961
- Eshwaran, L. Kazimirsky, G. and Byk, G. New Biocompatible Nanohydrogels of Predefined Sizes for Complexing Nucleic Acids. Pharmaceutics(IF:5.4, JCR, Q1) 2023, 15(2), 332. https://doi.org/10.3390/pharmaceutics15020332
- Gerardo Byk and Eswaran Lakshmanan " Preparation of biocompatible nanoparticles and uses thereof for drug and gene delivery" US Provisional Patent Application No. 63/378,071 (2/10/2022)
- Palakkal, S., Longviniuk, D., Byk, G. Tuning the size and hydrophobicity of nanohydrogels exploiting a self-assembly assisted polymerization mechanism for controlled drug delivery. Journal of Nanoparticle Research (2020) 22, 354.
- Massarano, T.; Mazir, A.; Lavi, R.; Byk, G. Solid-Phase Multicomponent Synthesis of 3-Substituted Isoindolinones Generates New Cell-Penetrating Probes as Drug Carriers. ChemMedChem (2020), 15(10), 833-838.
- Turdiev, A.; Filiutovich, O.; Mirkin, F.; Byk, G. A peptide from Testudo horsfieldii tortoise spleen as a potential helper for reducing acute radiation syndrome. J. Pept. Science (2019), 25(9)
- Shtriker E., Bretler S., Munder A., Byk G., Cohen G., Kolitz-Domb M., Gruzman A. Hydrogel nanoparticles covered by neuroligin-2-derived peptide-protected β cells under oxidative stress and increase their proliferation. J Nanoparticle Res. (2018) 20, 221.
- Gelman, M. ; Massarano, T; Lavi, R. ; Byk, G. Novel MCR4 multicomponent reaction: synthesis of biologically active analogs of staurosporine. Current Organic Chemistry (2018) 22, 505-517
- Byk, G. New Biocompatible Nanoparticles: Multistep chemical modifications and biological applications. Journal of Nanomedicine & Nanotechnology (2017) 8, 41-42.
- Byk G., Khandadash, R. ; Weiss A; Ebenstein Y; Gothilf, A andMachtey, V. Matrix Assisted peptide synthesis on new biocompatible nanoparticles. Chemistry Today (2015) 33(2), 26-29.
- Khandadash, R. ; Machtey, V.; Sheiner, I.; Gotilied, H.; Gothilf, A.; Ebenstein Y.; Weiss A. and Byk G. Novel biocompatible hydrogel nanoparticles: Generation and size-tuning of nanoparticles by the formation of micelle templates obtained from thermo-responsive monomers mixtures. Journal of Nanoparticle Research (2014) 16(12), 1-18.
- Khandadash, Raz; Machtey, Victoria; Weiss, Aryeh; Byk, Gerardo. Matrix-assisted peptide synthesis on nanoparticles. Journal of Peptide Science (2014), 20(9), 675-679.
- Byk, Gerardo; Cohen-Ohana, Mirit; Mirkin, Fiana: Lipoplexes and polyplexes: from gene delivery to gene expression. Pan Stanford Series on Biomedical Nanotechnology (2013), 4(Nanotechnology for the Delivery of Therapeutic Nucleic Acids), 1-25.
- Burkovitz, Anat; Leiderman, Olga; Sela-Culang, Inbal; Byk, Gerardo; Ofran, Yanay. Computational Identification of Antigen-Binding Antibody Fragments. Journal of Immunology (2013), 190(5), 2327-2334.
- Hagit Elhawi, Hadar Eini, Amos Douvdevani and Gerardo Byk. Improved Methods for Thermal Rearrangement of Alicyclic α-Hydroxyimines to α-Aminoketones. Synthesis of Ketamine Analogues as Antisepsis Candidates Molecules 2012, 17, 6784-6807.
- Maldiney, T., Byk, G., Wattier, N., Seguin, J., Khandadash, R., Bessodes, M., Richard, C., Scherman, D. Synthesis and functionalization of persistent luminescence nanoparticles with small molecules and evaluation of their targeting ability. Int. J. Pharmaceutics, (2012), 423, 102-107.
- Khandadash, R., Partouche, S., Weiss, A., Margel, S. and Byk, G. A Fully Synthetic Phage- Like System II: Synthesis and Live Cell Screening of Combinatorial Libraries of Peptides on Sub-Cellular Sized Microspheres. The Open Optics Journal, (2011) 5, 17-27.
- Byk G.; Machtey V.; Khandadash R. A Novel Dimension for Merrifield Synthesis: NPPS, Nano-Particle-Peptide-Synthesis. BIOPOLYMERS (2011), 96, 449-450.
- Khandadash R.; Machtey V.; Weiss A. and Byk, G. A Nanometric Platform for Synthesis and Biological Evaluation of Peptides. BIOPOLYMERS (2011) 96, 451-452.
- Machtey, V., Gottlieb, H. and Byk, G. Total synthesis of structures proposed for quinocitrinines A and B and their analogs. Microwave energy as efficient tool for generating heterocycles. ARKIVOC, 2011, IX, 308-324.
- Byk, G., Partouche, S., Weiss, A. Margel, S. and Khandadash, R. Fully Synthetic Phage-Like System for Screening Mixtures of Small Molecules in Live Cell. J. Comb. Chem. 2010, 12 (3), 332–345.
- Mirkin F, Eini H, Douvdevani A, Byk G. New degradable cationic peptides for modulated gene delivery. Adv Exp Med Biol. (2009) 611:245-6. Peptides for Youth, Emmanuel Escher, William D. Lubell, Susan Del Valle Edt. PEPTIDES FOR YOUTH V611, 245-246 (2009).
- Kun H., Byk G., Mastai Y. Effects of antifreeze protein fragments on the properties of model membranes. Adv Exp Med Biol. (2009) 611:85-6.
- Byk, G. Mozes, T. Tetramic acid peptide derivatives as cytotoxyc compounds. Biopolymers (2009) 9(4), 339-339.
- Khandadash, R., Partouche, S., Margel, S. and Byk, G. Towards a fully synthetic “phage-display like” system for high-throughput screening. J. Pept. Sci. (2008) 14( 8), 139.
- Davidov G., Mozes T., Khandadash R, Diestel R., Sasse F., Byk G. Versatile methods for synthesizing acyl-tetramic acids peptide analogs . J. Pept. Sci (2008) 14( 8), 59 .
- Gerardo Byk, Mirit Cohen-Ohana and Daniel Reichman. Fast and Versatile Microwave-Assisted Intramolecular Heck Reaction in Peptide Macrocyclization Using Microwave Energy. Biopolymers 2006, 81, 274-282.
- Gerardo Byk and Eihab Kabha. A Solid-Supported Stereoselective Multicomponenent Reaction: One-Pot Generation of Three Asymmetric carbons. SYNLETT, 2006, 5, 747-749.
- Gerardo Byk, David Visnovezky and Daniel Raichman. Fast and Versatile Backbone Modifications and Peptide Macrocyclization Using Microwave Energy. Biopolymers Pept. Sci. 2005, 80, 531.
- Gerardo Byk, Tamar Tennenbaum, Shlomo Margel and Shirly Partush. Towards in vivo screening of small molecules: Micron sized monodispersed polystyrene for synthesis, tagging and biological screening of small molecules. Biopolymers Pept. Sci. 2005, 80, 597-598.
- Julia Mazar, Boris Rogachev, Gad Shaked, Nadav Y. Ziv, David Czeiger,Cidio Chaimovitz, Moshe Zlotnik, Igor Mukmenev, Gerardo Byk and Amos Douvdevani. Involvement of Adenosine in the Anti-inflammatory Action of Ketamine. Anesthesiology (2005)102(6), 1174-1181.
- Gerardo Byk and Eihab Kabha. Anomalous regiselective MCR4 Biginelli reaction II: One pot parallel synthesis of spiro heterobicyclic aliphatic rings. Journal of Combinatorial Chemistry (2004), 6(4), 596-603.
- Alexander Aizikovich, Vladimir Kuznetsov, Sofia Gorohovsky, Amalia Levy, Simha Meir, Gerardo Byk and Garry GellermanA new application of diphenylphosphorylazide (DPPA) reagent: convenient transformations of quinolin-4-one, pyridin-4-one and quinazolin-4-one derivatives into 4-azido and 4-amino counterparts. Tetrahedron Lett.,(2004) 45, 4241-4243
- Vladimir Kuznetsov, Sofia Gorohovsky, Amalia Levy, Simcha Meir, Vladimir Shkoulev, Naim Menashe, Moshe Greenwald, Alexander Aizikovich, Dror Ofer, Gerardo Byk and Garry Gellerman. Approaches for introducing high molecular diversity in scaffolds: Fast Parallel Synthesis of Highly Substituted 1H-quinolin-4-one Libraries. Molecular Diversity, (2004), 8(4), 437-448.
- Gerardo Byk and Eihab Kabha. Regioselective MCR4 reaction: One pot synthesis of spiro heterobicyclic aliphatic rings generated from natural amino acids. Biopolymers, 71(3), 235 (2003).
- Gerardo Byk, Elisha Haas, Varda Itah and Inbar Rofman. A versatile method for fast and selective introduction of multiple probes into peptides using solid phase synthesis. Applications to biophysical models. Biopolymers 71(3), 470 (2003).
- Tom Einbinder, Yuval Sufaro, Igor Yusim, Gerardo Byk, Jutta Passlick-Deetjen, Cidio Chaimovitz and Amos Douvdevani: Correction of anemia in uremic mice by genetically modified peritoneal mesothelial cells. Kidney International, Vol. 63 (2003), 2103-2112.
- Sofia Gorohovsky, Simha Meir, Vladimir Shkoulev, Gerardo Byk and Garry Gellerman: A Facile Two-step Synthesis of Novel ring-A double substituted Tryptophan Building Blocks for Combinatorial Chemistry. SYNLETT 10, 1411-1414 (2003).
- Francoise Leclercq, Mirit Cohen-Ohana, Nathalie Mignet, Andrea Sbarbati, Jean Herscovici, Daniel Scherman and Gerardo Byk. Design, Synthesis and Evaluation of Gadolinium Cationic Lipids As Tools for Biodistribution Studies of Gene Delivery Complexes. Bioconjugate Chem. 14, 112-119 (2003).
- Nathalie Mignet, Gerardo Byk, Barbara Wetzer and Daniel Scherman. DNA complexes with reducible cationic lipid for gene transfer. Methods in Enzymology 373, 357-69, (2003).
- Gerardo Byk. Cationic Lipid Based Gene Delivery, in: Pharmaceutical Perspectives of Nucleic Acid Based Therapy. Edited by Ram I. Mahato and Sung Wan Kim Part 3(15), pg 302-333 (2002).
- Carriere, Marie; Tranchant, Isabelle; Niore, Pierre-Antoine; Byk, Gerardo; Mignet, Nathalie; Escriou, Virginie; Scherman, Daniel; Herscovici, Jean. Optimization of cationic lipid mediated gene transfer: structure-function, physico-chemical, and cellular studies. Journal of Liposome Research 12(1 & 2), 95-106, (2002)
- Marks, Vered; Gottlieb, Hugo E.; Melman, Artem; Byk, Gerardo; Cohen, Shmuel; Biali, Silvio E. Journal of Organic Chemistry, (2001) 66(20), 6711-6718: Polyethylated Aromatic Rings: Conformation and Rotational Barriers of 1,2,3,4,5,6,7,8-Octaethylanthracene, 1,2,3,4,6,7,8-Heptaethylfluorene, and 1,2,3,4,5,6,7,8-Octaethylfluorene.
- Wetzer, Barbara; Byk, Gerardo; Frederic, Marc; Airiau, Marc; Blanche, Francis; Pitard, Bruno; Scherman, Daniel. Reducible cationic lipids for gene transfer. Biochem. J. (2001), 356(3), 747-756.
- Raspaud, Eric; Pitard, Bruno; Durand, Dominique; Aguerre-Chariol, Olivier; Pelta, Juan; Byk, Gerardo; Scherman, Daniel; Livolant, Francoise. Polymorphism of DNA/Multi-cationic Lipid Complexes Driven by Temperature and Salts. J. Phys. Chem. B (2001), 105(22), 5291-5297.
- Byk, Gerardo; Gottlieb, Hugo E.; Herscovici, Jean; Mirkin, Fiana. New Regioselective Multicomponent Reaction: One Pot Synthesis of Spiro Heterobicyclic Aliphatic Rings J. Comb. Chem. (2000), 2(6), 732-735.
- Byk, Gerardo; Wetzer, Barbara; Frederic, Marc; Dubertret, Catherine; Pitard, Bruno; Jaslin, Gabrielle; Scherman, Daniel. Reduction-Sensitive Lipopolyamines as a Novel Nonviral Gene Delivery System for Modulated Release of DNA with Improved Transgene Expression. J. Med. Chem. (2000), 43(23), 4377-4387.
- Byk, Gerardo; Scherman, Daniel. Genetic chemistry: tools for gene therapy coming from unexpected directions. Drug Dev. Res. (2000), 50(3/4), 566-572.
- Soto, Javier; Bessodes, Michel; Pitard, Bruno; Mailhe, Philippe; Scherman, Daniel; Byk, Gerardo. Non-electrostatic complexes with DNA: Towards novel synthetic gene delivery systems. Bioorg. Med. Chem. Lett. (2000), 10(9), 911-914.
- Frederic, Marc; Scherman, Daniel; Byk, Gerardo. Introduction of cyclic guanidines into cationic lipids for non-viral gene delivery. Tetrahedron Lett. (2000), 41(5), 675-679.
- Neves, Carole; Byk, Gerardo; Escriou, Virginie; Bussone, Florence; Scherman, Daniel; Wils, Pierre. Novel Method for Covalent Fluorescent Labeling of Plasmid DNA That Maintains Structural Integrity of the Plasmid. Bioconjugate Chem. (2000), 11(1), 51-55.
- Neves, C.; Escriou, V.; Byk, G.; Scherman, D.; Wils, P. Intracellular fate and nuclear targeting of plasmid DNA. Cell Biol. Toxicol. (1999), 15(3), 193-202.
- Neves, Carole; Byk, Gerardo; Scherman, Daniel; Wils, Pierre. Coupling of a targeting peptide to plasmid DNA by covalent triple helix formation. FEBS Lett. (1999), 453(1, 2), 41-45.
- Schwartz, B.; Ivanov, M-A.; Pitard, B.; Escriou, V.; Rangara, R.; Byk, G.; Wils, P.; Crouzet, J.; Scherman, D. Synthetic DNA-compacting peptides derived from human sequence enhance cationic lipid-mediated gene transfer in vitro and in vivo. Gene Ther. (1999), 6(2), 282-292.
- Ciolina, Carole; Byk, Gerardo; Blanche, Francis; Thuillier, Vincent; Scherman, Daniel; Wils, Pierre. Coupling of Nuclear Localization Signals to Plasmid DNA and Specific Interaction of the Conjugates with Importin. Bioconjugate Chem. (1999), 10(1), 49-55.
- Byk, Gerardo; Scherman, Daniel. Novel cationic lipids for gene delivery and gene therapy. Expert Opin. Ther. Pat. (1998), 8(9), 1125-1141.
- Byk, Gerardo; Soto, Javier; Mattler, Christophe; Frederic, Marc; Scherman, Daniel. Novel non-viral vectors for gene delivery: synthesis of a second-generation library of mono-functionalized poly-(guanidinium)amines and their introduction into cationic lipids. Biotechnol. Bioeng. (1998), 61(2), 81-87.
- Byk, Gerardo; Dubertret, Catherine; Escriou, Virginie; Frederic, Marc; Jaslin, Gabrielle; Rangara, Ravi; Pitard, Bruno; Crouzet, Joel; Wils, Pierre; Schwartz, Bertrand; Scherman, Daniel. Synthesis, Activity, and Structure-Activity Relationship Studies of Novel Cationic Lipids for DNA Transfer. J. Med. Chem. (1998), 41(2), 224-235.
- Escriou, Virginie; Ciolina, Carole; Lacroix, Florence; Byk, Gerardo; Scherman, Daniel; Wils, Pierre. Cationic lipid-mediated gene transfer: effect of serum on cellular uptake and intracellular fate of lipopolyamine/DNA complexes. Biochim. Biophys. Acta (1998), 1368(2), 276-288.
- Byk, Gerardo; Dubertret, Catherine; Schwartz, Bertrand; Frederic, Marc; Jaslin, Gabrielle; Rangara, Ravi; Scherman, Daniel. Novel nonviral vectors for gene delivery: synthesis and applications. Lett. Pept. Sci. (1997), 4(4/5/6), 263-267.
- Pitard, Bruno; Aguerre, Olivier; Airiau, marc; Lachages, Anne-Marie; Boukhnikachvili, Tsiala; Byk, Gerardo; Dubertret, Catherine; Herviou, Christian; Scherman, Daniel; Mayaux, Jean-Francois; Crouzet, Joel. Virus-sized self-assembling lamellar complexes between plasmid DNA and cationic micelles promote gene transfer. Proc. Natl. Acad. Sci. U. S. A. (1997), 94(26), 14412-14417.
- Byk, Gerardo; Frederic, Marc; Scherman, Daniel. One pot synthesis of unsymmetrically functionalized polyamines by a solid phase strategy starting from their symmetrical polyamine-counterparts. Tetrahedron Lett. (1997), 38(18), 3219-3222.
- Byk, Gerardo; Lelievre, Yves; Duchesne, Marc; Clerc, Francois F.; Scherman, Daniel; Guitton, Jean Dominique. Synthesis and conformational analysis of peptide inhibitors of farnesyltransferase. Bioorg. Med. Chem. (1997), 5(1), 115-124.
- Byk, Gerardo and Scherman Daniel. Synthesis of novel (N-farnesyl)amino acids and their incorporation into peptides. Int. J. Peptide Protein Res. (1996), 47, 333-339.
- Bitan, Gal; Zeltser, Irena; Byk, Gerardo; Halle, David; Mashriki, Yaffa; Gluhov, Evgenia V.; Sukhotinsky, Inna; Hanani, Menachem; Selinger, Zvi; Gilon, Chaim. Backbone cyclization of the C-terminal part of substance P. Part 1: The important role of the sulfur in position 11. J. Pept. Sci. (1996), 2(4), 261-269.
- Byk, Gerardo; Halle, David; Zeltser, Irena; Bitan, Gal; Selinger, Zvi; Gilon, Chaim. Synthesis and Biological Activity of NK-1 Selective, N-Backbone Cyclic Analogs of the C-Terminal Hexapeptide of Substance P. J. Med. Chem. (1996), 39(16), 3174-3178.
- Byk, Gerardo; Duchesne, Marc; Parker, Fabienne; Lelievre, Yves; Guitton, Jean D.; Clerc, Francois F.; Becquart, Jerome; Tocque, Bruno; Scherman, Daniel; et al. Local constrained shifty pseudopeptides inhibitors of ras-farnesyl transferase. Bioorg. Med. Chem. Lett. (1995), 5(22), 2677-82.
- Grdadolnik, Simona Golic; Mierke, Dale F.; Byk, Gerardo; Zeltser, Irena; Gilon, Chaim; Kessler, Horst. Comparison of the Conformation of Active and Nonactive Backbone Cyclic Analogs of Substance P as a Tool To Elucidate Features of the Bioactive Conformation: NMR and Molecular Dynamics in DMSO and Water. J. Med. Chem. (1994), 37(14), 2145-52.
- Byk, Gerardo and McGregor, John L. Role of Type I Motif CSVTCG in the Thronbispondin Interaction with other Adhesive Proteins: Effect on Platelet Aggregation. Thrombosis-Haemostasis (1993) 69, 1009.
- Byk, Gerardo; Gilon, Chaim. Building units for N-backbone cyclic peptides. 1. Synthesis of protected N-(-aminoalkylene)amino acids and their incorporation into dipeptide units. J. Org. Chem. (1992), 57(21), 5687-92.
- Saulitis, Juris; Mierke, Dale F.; Byk, Gerardo; Gilon, Chaim; Kessler, Horst. Conformation of cyclic analogs of substance P: NMR and molecular dynamics in dimethyl sulfoxide. J. Am. Chem. Soc. (1992), 114(12), 4818-27.
- Gilon, Chaim; Halle, David; Chorev, Michael; Selinger, Zvi; Byk, Gerardo. Backbone cyclization: a new method for conferring conformational constraint on peptides. Biopolymers (1991), 31(6), 745-50.
- Byk, Gerardo and Kabha, Eihab, Regioselective MCR4 reaction: One Pot Synthesis of Spiro Hetetrobicyclic Aliphatic Rings generated from Natural Amino Acids: in Peptide Revolution: Genomics, Proteomics &Therapeutics Michael Chorev & Tomi K. Sawyer (Editors). American Peptide Society (2003) pg 104-105.
- Byk, Gerardo; Haas, Elisha; Ittah, Vardah and Rofman Inbar: A Versatile Method for Fast and Selective Introduction of Multiple Probes into Peptides Using Solid Phase Synthesis-Application to Biophysical Models. In Peptide Revolution: Genomics ,Proteomics & Therapeutics Michael Chorev &Tomi K. Sawyer (Editors). American Peptide Society (2003) pg 91-92.
- Byk, Gerardo; Engel, Dikla; Ittah, Vardah; Haas, Elisha: Peptide models for studying autonomous folding of subdomain sections of protein molecules. In Peptides: The Wave of the Future, Proceedings of the Second International and the Seventeenth American Peptide Symposium, San Diego, CA, United States, June 9-14, 2001 (2001), 398-399.
- Byk, Gerardo; Dubertret, Catherine; Schwartz, Bertrand; Frederic, Marc; Jaslin, Gabrielle; Scherman, Daniel. Synthesis, characterization and use of novel nonviral vectors for gene delivery. Editor(s): Tam, James P.; Kaumaya, Pravin T. P. Pept. Proc. Am. Pept. Symp., 15th (1999), Meeting Date 1997, 823-824. Publisher: Kluwer, Dordrecht, Netherlands.
- Byk, Gerardo; Frederic, Marc; Scherman, Daniel. Unsymmetrically functionalized polyamine libraries by a solid phase strategy starting from their symmetrical polyamine-counterparts. Editor(s): Tam, James P.; Kaumaya, Pravin T. P. Pept. Proc. Am. Pept. Symp., 15th (1999), Meeting Date 1997, 47-48. Publisher: Kluwer, Dordrecht, Netherlands.
- Bitan, G.; Byk, G.; Mashriki, Y.; Hanani, M.; Halle, D.; Selinger, Z.; Gilon, C. Structure-activity relationship studies of new backbone-cyclic substance P analogs. Editor(s): Kaumaya, Pravin T. P.; Hodges, Robert S. Pept.: Chem., Struct. Biol., Proc. Am. Pept. Symp., 14th (1996), Meeting Date 1995, 697-698. Publisher: Mayflower Scientific, Kingswinford, UK.
- Byk, G.; Burns, C.; Duchesne, M.; Parker, F.; Lelievre, Y.; Guitton, J. D.; Clerc, F. F.; Commercon, A.; Tocque, B.; et al. Constrained pseudopeptides as inhibitors of Ras-farnesyl transferase: Structure-activity relationship studies. Editor(s): Kaumaya, Pravin T. P.; Hodges, Robert S. Pept.: Chem., Struct. Biol., Proc. Am. Pept. Symp., 14th (1996), Meeting Date 1995, 213-214. Publisher: Mayflower Scientific, Kingswinford, UK.
- Byk, G.; Scherman, D. Synthesis of backbone-modified (N-farnesyl) amino acids and their incorporation into peptides. Editor(s): Kaumaya, Pravin T. P.; Hodges, Robert S. Pept.: Chem., Struct. Biol., Proc. Am. Pept. Symp., 14th (1996), Meeting Date 1995, 107-108. Publisher: Mayflower Scientific, Kingswinford, UK.
- Byk, Gerardo; McGregor, John L. Peptide analogs derived from the thrombospondin type I repeat (CSVTCG) inhibit platelet aggregation. Editor(s): Schneider, Conrad H.; Eberle, Alex N. Pept. 1992, Proc. Eur. Pept. Symp., 22nd (1993), Meeting Date 1992, 827-8. Publisher: ESCOM, Leiden, Neth.
- Gilon, Chaim; Zeltser, Irena; Rashti-Bahar, Vered; Muller, Dan; Bitan, Gal; Halle, David; Bar-Akiva, Giora; Selinger, Zvi; Byk, Gerardo. Backbone cyclization as a tool for imposing conformational constraint on peptides. Editor(s): Yanaihara, Noboru. Pept. Chem. 1992, Proc. Jpn. Symp., 2nd (1993), Meeting Date 1992, 482-5. Publisher: ESCOM, Leiden, Neth.
- Gilon, C.; Halle, D.; Chorev, M.; Selinger, Z.; Byk, G.. SAR studies of cycloseptide: Effects of cyclization and charge at position 6. Editor(s): Smith, John A.; Rivier, Jean E. Pept.: Chem. Biol., Proc. Am. Pept. Symp., 12th (1992), Meeting Date 1991, 476-7. Publisher: ESCOM, Leiden, Neth.
- Gilon, Chaim; Halle, David; Chorev, Michael; Selinger, Zvi; Goldshmith, Rafi; Byk, Gerardo. Backbone-to-end cyclic tachykinins: a new approach to conformationally restricted peptides. Editor(s): Giralt, Ernest; Andreu, David. Pept. 1990, Proc. Eur. Pept. Symp., 21st (1991), Meeting Date 1990, 404-6. Publisher: ESCOM Sci. Publ., Leiden, Neth.
- Byk, Gerardo; Gur, Erez; Halle, David; Chorev, Michael; Selinger, Zvi; Gilon, Chaim. A new route to prepare conformationally restricted cyclic peptides as demonstrated by a potent NK-1 selective substance P analog. Editor(s): Rivier, Jean E.; Marshall, Garland R. Pept.: Chem., Struct. Biol., Proc. Am. Pept. Symp., 11th (1990), Meeting Date 1989, 984-5. Publisher: ESCOM Sci. Pub., Leiden, Neth.
Courses
Organic Chemistry Laboratory II
Advanced Organic chemistry
Graduate Students Seminar: Bioorganic Chemistry Group
Patents
Patents
-
Gerardo Byk and Eswaran Lakshmanan " Preparation of biocompatible nanoparticles and uses thereof for drug and gene delivery" US Provisional Patent Application No. 63/378,071
-
Gerardo Byk, Ilya Olevsko, Adi Salomon. "Highly efficient fluorescent thin films" US 63/478,204. Jan. 3, 2023.
- Byk, Gerardo; Dubertret, Catherine; Pitard, Bruno; et al. Transfecting compounds which are sensitive to reducing conditions, pharmaceutical compositions containing them and their applications Patent Number: US 6521252 Patent Assignee: Aventis Pharma S.A., Antony, France Inventor(s): Official Gazette of the United States Patent and Trademark Office Patents Volume: 1267 Issue: 3 Published: Feb. 18, 2003 .
- Scherman, Daniel; Bessodes, Michel; Pitard, Bruno; Soto, Javier; Byk, Gerardo. Oligobenzimidazole derivatives and their use as DNA transfection agents. (Aventis Pharma S.A., Fr.). PCT Int. Appl. (2001), 56 pp. Patent written in French. Application: WO 2000-2000FR3087 20001106. Priority: FR 99-13934 19991105; US 2000-174648 20000105. CAN 134:336672 AN 2001:338501
- Byk, Gerardo; Frederic, Marc; Hofland, Hans; Schermann, Daniel. Amidine-containing lipopolyamines, their synthesis and use in transfection. (Rhone-Poulenc Rorer S.A., Fr.). PCT Int. Appl. (1999), 83 pp. Patent written in French. Application: WO 99-FR740 19990330. Priority: FR 98-4121 19980402; US 98-85845 19980518. CAN 131:267946 AN 1999:659366
- Byk, Gerardo; Dubertret, Catherine; Pitard, Bruno; Scherman, Daniel. Transfecting compositions comprising DNA-binding, disulfide bond-containing compounds and their use in gene therapy. (Rhone-Poulenc Rorer S.A., Fr.). PCT Int. Appl. (1999), 51 pp. CODEN: PIXXD2 WO 9938821 A2 19990805 . Patent written in French. Application: WO 99-FR162 19990128. Priority: FR 98-1065 19980130; US 98-77026 19980306. CAN 131:140459 AN 1999:495250
- Byk, Gerardo; Dubertret, Catherine; Scherman, Daniel. Preparation of lipopolyamine-related compounds for transferring nucleic acids into cells. (Rhone-Poulenc Rorer S.A., Fr.). PCT Int. Appl. (1998), 80 pp. Patent written in French. Application: WO 98-FR1041 19980525. Priority: FR 97-6549 19970528. CAN 130:38711 AN 1998:793121
- Byk, Gerardo; Scherman, Daniel; Schwartz, Bertrand; Dubertret, Catherine. Lipopolyamines as transfection agents and pharmaceutical uses thereof. (Rhone-Poulenc Rorer S.A., Fr.; Byk, Gerardo; Scherman, Daniel; Schwartz, Bertrand; Dubertret, Catherine). PCT Int. Appl. (1997), 81 pp. Patent written in French. Application: WO 96-FR1774 19961108. Priority: FR 95-13490 19951114. CAN 127:46039 AN 1997:440187
- Byk, Gerardo; Scherman, Daniel; Schwartz, Bertrand. Nucleic acid-containing compositions containing transfecting agents and nucleic acid condensing agents and their use in transfection. (Rhone-Poulenc Rorer S.A., Fr.). PCT Int. Appl. (1996), 53 pp. CODEN: PIXXD2 WO 9625508 A1 19960822 Patent written in French. Application: WO 96-FR248 19960215. Priority: FR 95-1865 19950217. CAN 125:240223 AN 1996:609959
- Byk, Gerardo; Dubertret, Catherine; Scherman, Daniel. Lipopolyamines as transfection agents and pharmaceutical uses thereof. (Rhone-Poulenc Rorer S.A., Fr.). PCT Int. Appl. (1996), 36 pp. CODEN: PIXXD2 WO 9617823 A1 19960613 Patent written in French. Application: WO 95-FR1595 19951204. Priority: FR 94-14596 19941205. CAN 125:160332 AN 1996:506088
- Gilon, Chaim; Zelinger, Zvi; Byk, Gerardo. Preparation of backbone cyclic peptides as drugs and pharmaceutical compositions containing them. (Hebrew University of Jerusalem, Israel). Eur. Pat. Appl. (1993), 48 pp. CODEN: EPXXDW EP 564739 A2 19931013 Patent written in English. Application: EP 92-309016 19921002. Priority: IL 91-99628 19911020. CAN 121:206022 AN 1994:606022
Research Group
Current Group (2021)
Gila Kasimirsky laboratory manager
Eshwaran Lakshmanan, PhD student
Shaul Cemal, PhD student
Sinyal Abu-Rabiah, MSc student
Danit Segal, MSc student
Former students
Eshwaran Lakshmanan, PhD
Sarin Palakkal, PhD
Raz Khandadash, PhD
Victoria Machtey, PhD
Eihab Kabha, PhD
Hagit Elkawi, PhD
Shirli Partouche, PhD
Fiana Mirkin, PhD
Mirit Cohen Ohana, PhD
Shaul Cemal, MSc
Alexandra Mazir, MSc
Mati Bardosh Gelman MSc
Tlalit Massarano, MSc
Vladimir Gubri, MSc
Raz Khandadash, MSc
Daniel Raichman, MSc
Tamar Mozes, MSc
Gali Davidov, MSc
Dina Spivak, MSc
Dikla Engel, MSc
Maya Many, MSc
Fiana Mirkin MSc
Media
Last Updated Date : 19/11/2024



